

In 1897, Thomson showed that cathode rays were composed of previously unknown negatively charged particles (now called electrons), which he calculated must have bodies much smaller than atoms and a very large charge-to-mass ratio. Sir Joseph John Thomson OM PRS (18 December 1856 – 30 August 1940) was a British physicist and Nobel Laureate in Physics, credited with the discovery of the electron, the first subatomic particle to be discovered. Thomson: Computational Chemistry and Gas Discharge Experiments Thomson demonstrated that cathode rays are particles in nature.Owens College (now the University of Manchester) Thomson's determination of the charge to mass ratio of cathode rays settled the debate of the nature of cathode rays. Therefore, they have mass and are particles in nature.

Observation: when the glass paddle wheel was struck by the cathode ray, it rotated and moved towards the cathode.What was done: a glass paddle wheel that could move and rotate freely was placed in the path of the cathode ray.Conclusion: cathode rays are streams of negatively charged particles.In the presence of a magnetic field, the path of the cathode ray was deflected in a direction that was consisted with a negatively charged mass. Observation: in the presence of an electric field, the path of the cathode ray was deflected towards the positively charged plate.What was done: a metal plate coated with fluorescent material was used to visualise the trajectory of a cathode ray. The cathode ray was passed through a uniform electric field and magnetic field (in separate experiments).However, this observation could also be produced by particles.Ĭathode Ray Tubes Containing Electric and Magnetic Fields Some scientists argued that since waves such as light can produce a similar observation, cathode rays are wave in nature. Conclusion: Cathode rays travel in a straight line and can cast a shadow.Observation: a shadow of the maltese cross was formed directly behind the anode.

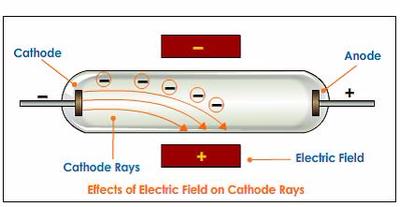
What was done: An anode in the shape of a maltese cross was placed in the path of the cathode ray.It emanates from the cathode and travels in a straight line They are identical, regardless of material used These experiments produced conflicting conclusions on the nature of cathode rays: some suggested that cathode rays were waves and others suggested that they were particles. Particle vs Wave Nature of CathodesĪfter cathode ray was demonstrated, further experiments were performed to investigate its nature and identity. It is important to keep in mind that at the time when cathode rays were observed, scientists were not aware of the existence of electrons. In a near-vacuum setting where there are few to none air molecules, these electrons can travel unimpeded.Ī simple representation of a cathode ray tubeĪ cathode ray was the name given to the observation of these electrons when they were incident on the glass coated with fluorescent material behind the cathode. The high potential difference causes electrons to move from the anode to the cathode. Similar to a discharge tube, a cathode ray tube consists of two electrodes connected to a high potential difference. The positive electrode is the anode, and the negative electrode is the cathode. Cathode rays were produced in partially evacuated discharged tubes called Crookes tubes (cathode ray tubes).Ī simple representation of a gas discharge tube


 0 kommentar(er)
0 kommentar(er)
